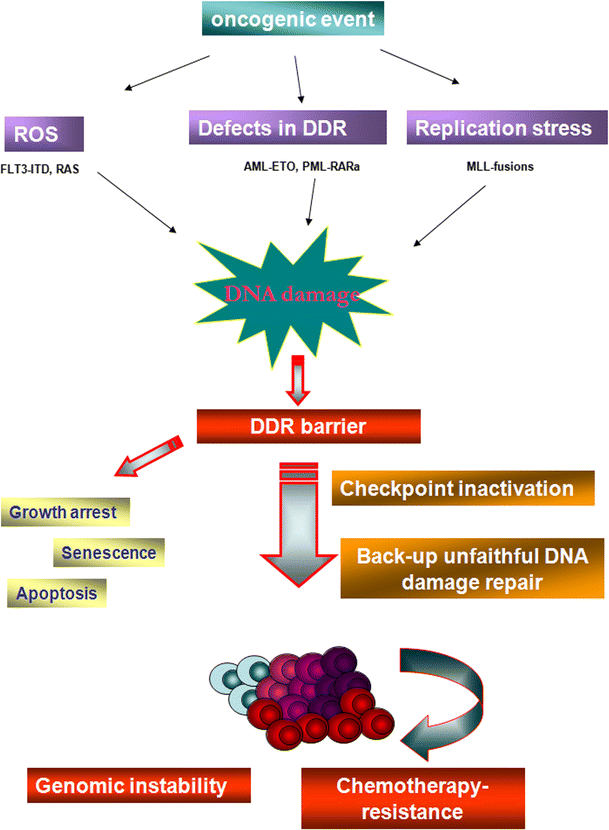
DNA damage accumulation and repair defects in acute myeloid leukemia Biology Diagrams The accumulation of these DNA damage can be particularly deleterious in postmitotic cells such as neurons, which are not self-renewed through cell proliferation (Coppede and Migliore 2009). It is believed that DNA damage can promote the age-associated neurodegenerative process such as Alzheimer's disease
Overall, these lines of evidence suggest DNA damage accumulation in aged skin stem cells and raises the question of how these stem cells address this burden. HFSCs, as many of the tissue specific stem cells, are more resistant to DNA damage induced apoptosis compared to more differentiated cells (Solanas et al. 2017; Gutierrez-Martinez et al. 2018 It should be noted that these techniques mainly provide snapshots of DNA damage accumulation and not direct assessments of DNA repair dynamics at specific sites. In the future, combining insights from these methods with those that allow for direct monitoring of DNA repair should reveal how DNA damage contributes to the decline in neuronal Recent studies have highlighted the role of DNA damage, particularly DNA double-strand breaks (DSBs), in the progression of neuronal loss in a broad spectrum of neurodegenerative diseases. In the present study, we tested the hypothesis that accumulation of DNA DSB plays an important role in AD pathogenesis.
Systemic DNA damage responses in aging and diseases Biology Diagrams
It is thought that DNA damage accumulation with aging results in loss of cellular functionality and ultimately degeneration of cells and tissues. Erroneous repair, however, can lead to mutations and chromosomal aberrations, which when affecting tumor suppressor genes, drive carcinogenesis. Alternatively, unrepaired DNA lesions can lead to cell The accumulation of DNA double-strand damage has been observed in aging mice and human cells (Sedelnikova et al., 2004a).In AD model mice, it has been observed that exploring new environments leads to increased DNA double-strand damage in several brain regions, especially those involved in spatial learning and memory pairs (Suberbielle et al., 2013), and elevated levels of γ-H2AX in Here we synthesize accumulating evidence that DNA damage affects most, if not all, aspects of the ageing phenotype, making it a potentially unifying cause of ageing. Targeting DNA damage and its mechanistic links with the ageing phenotype will provide a logical rationale for developing unified interventions to counteract age-related dysfunction

Accumulation of DNA damage has been observed in human and mouse HSCs as well as in muscle, intestinal, mesenchymal, neural, skin and germ stem cells 72. Accumulation of DNA damage may be particularly prevalent in the central nervous system owing to the low DNA repair capacity in postmitotic brain tissue. It is generally believed that the cumulative effects of the deleterious changes that occur in aging, mostly after the reproductive phase, contribute to species-specific rates of aging. Many factors contribute to aging, such as the time-dependent accumulation of macromolecular damage, including DNA damage. The integrity of the nuclear genome is essential for cellular, tissue, and organismal health. DNA damage is a constant threat because nucleic acids are chemically unstable under physiological conditions and vulnerable to

