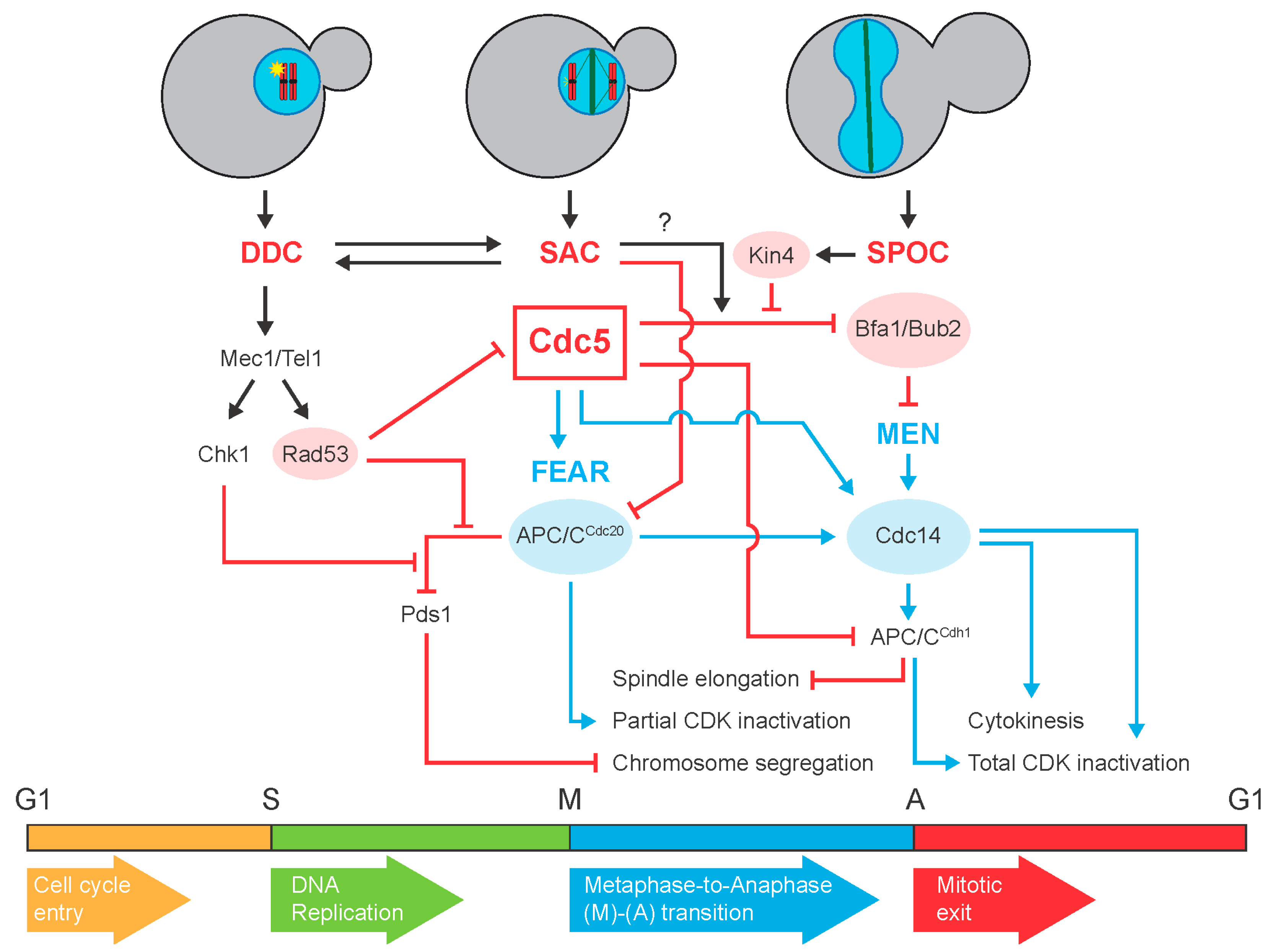
Targeted protein degradation in mammalian cells A promising avenue Biology Diagrams Mitotic exit is an important transition point that signifies the end of mitosis and the onset of new G1 phase for a cell, and the cell needs to rely on specific control mechanisms to ensure that once it exits mitosis, it never returns to mitosis until it has gone through G1, S, and G2 phases and passed all the necessary checkpoints. Many factors including cyclins, cyclin-dependent kinases Protein degradation removes proteins and hence their phosphorylation status, ensuring that mitotic exit continues in one direction and does not reverse (11, 12). However, currently only ∼170 proteins have been found to be targeted for degradation during mitotic exit ( 13 ), which although likely to be a significant underestimation, is only For example, only one Aurora kinase is present in yeast, whereas there are three in mammals, of which at least two are degraded during mitotic exit. Degradation of AurA starts soon after anaphase onset, and both its degradation and activity are required for assembly of a robust spindle midzone (Floyd et al., 2008; Lioutas and Vernos, 2013

The first step in mitotic exit is sister-chromatid separation, which is triggered, Hochstrasser, M. Ubiquitin-dependent protein degradation. Annu. Rev. Genet. 30, 405-439 (1996).

Mitotic exit Biology Diagrams
Abstract. We have found that key mitotic regulators show distinct patterns of degradation during exit from mitosis in human cells. Using a live-cell assay for proteolysis, we show that two of these regulators, polo-like kinase 1 (Plk1) and Aurora A, are degraded at different times after the anaphase-promoting complex/cyclosome (APC/C) switches from binding Cdc20 to Cdh1. Mitotic exit regulation involves inactivation of mitotic kinases and activation of counteracting protein phosphatases. The key mitotic exit phosphatase in budding yeast, Cdc14, is now well understood. As expected, given the observed enrichments of the Time Course and Mitotic Exit protein datasets, CCD proteins were highly enriched in cell cycle annotations, cell cycle-specific cellular

Mitotic exit is characterized by mitotic spindle breakdown, chromosome decondensation and reassembly of interphase structures, particularly the NE. Similarly, degradation of the F-box protein Tome-1 during G1 enables accumulation of the Wee1 inhibitory kinase during interphase [30]. Background: Degradation of the mitotic cyclins is a hallmark of the exit from mitosis. Induction of stable versions of each of the three mitotic cyclins of Drosophila, cyclins A, B, and B3, arrests mitosis with different phenotypes. We tested a recent proposal that the destruction of the different cyclins guides progress through mitosis.Results: Real-time imaging revealed that arrest a specific protein degradation signal that is required for the regulated destruction of the protein degradation rate. A degron can be a short amino acid sequence or a structural motif. Some central components Ub of the SAC, including Cdc20, contain multiple degrons. Hop1p, Rev7p, and MAD2 (HORMA) domain: functions as an adaptor for the